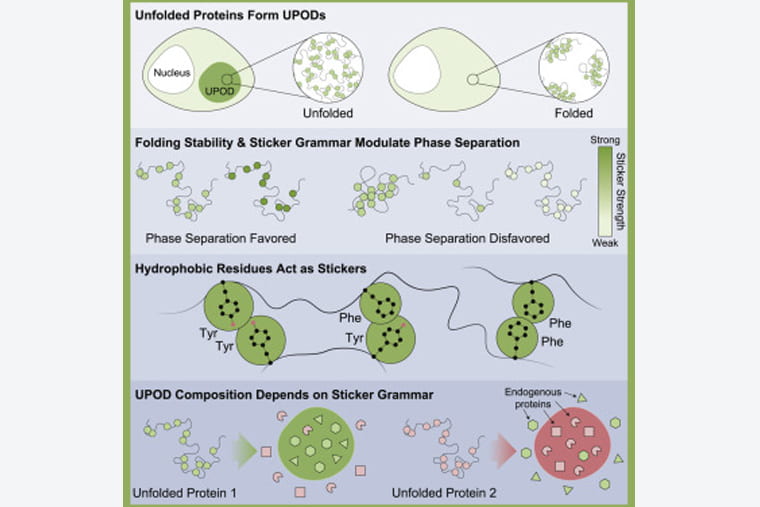
Unfolded proteins are unhealthy proteins. When found inside of cells, they are rounded up, identified, and destroyed. This is an important quality-control process, especially in the brain and the heart.
How these unfolded proteins are identified, however, has been a mystery. Now, research led by Kiersten Ruff, a senior research scientist in the lab of Rohit Pappu, PhD, the Gene K. Beare Distinguished Professor of biomedical engineering at Washington University in St. Louis’ McKelvey School of Engineering, has uncovered the rules that govern this process — and found that exceptions to these rules may play a role in dysfunctional cells.
The research was published in the journal Molecular Cell and was a collaborative effort between the labs of Pappu and Danny Hatters, professor of biochemistry at the University of Melbourne in Australia.
A healthy cell will begin cleaning out unfolded proteins once they have collected in clumps called, appropriately, unfolded protein deposits (UPODS). But researchers were not sure how these UPODs formed.
Ruff, Pappu said, came up with an idea.
“She had the brilliant insight that there likely was a combination of factors involved in driving the formation of UPODs,” Pappu said. “She reasoned that it could not just be unfolded proteins crossing some threshold concentration. There must be specific interactions that must be mediating UPOD formation.”
Ruff’s insight, driven by a close collaboration with members of the Hatters lab, led to the finding that the formation of UPODs requires the crossing of a threshold concentration in cells along with the presence of specific stickers — particular residues within proteins that drive them to stick to one another.