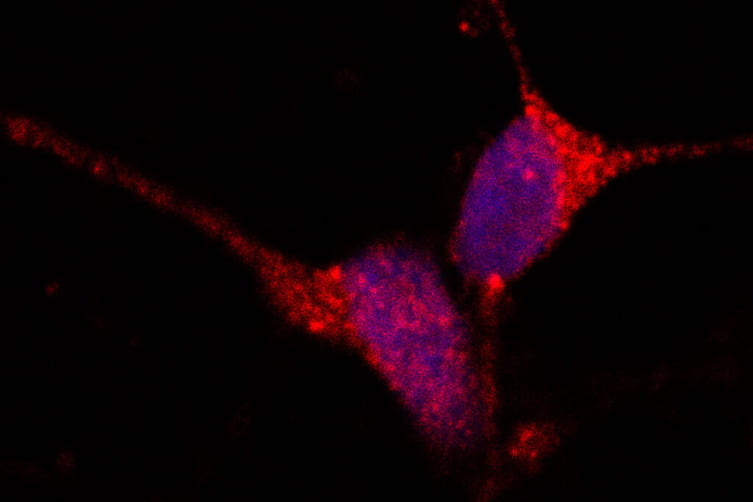
Under normal circumstances, tau protein is part of the brain’s infrastructure, important for stabilizing neurons into their proper shapes. But sometimes tau gets knotted up into tangles and turns toxic, injuring brain tissue and causing tauopathies, a group of brain diseases characterized by problems with learning, memory and movement. Alzheimer’s disease is the most common tauopathy, but the group also includes Parkinson’s disease, chronic traumatic encephalopathy (CTE) and several rare genetic conditions.
In search of ways to prevent these destructive tau tangles, researchers at Washington University School of Medicine in St. Louis have identified a key step in their development. Intervening at this step potentially could forestall the destructive cascade of events that results in brain damage, the researchers said. The findings are published Sept. 20 in the journal Molecular Psychiatry.
“Tauopathies are devastating diseases that have limited treatment options right now, and they all have this feature of tau aggregation,” said senior author Celeste Karch, PhD, a professor of psychiatry. “We’ve been thinking for a long time about whether there are factors that impact that common process of tau aggregation and if so, whether we could target those factors as a novel approach to treatment. These findings move us one step closer to finding a way to intervene and stop the process of tau aggregation that leads to dementia.”
First author Reshma Bhagat, PhD, a postdoctoral researcher, came up with the idea of looking for such factors among a group of RNA molecules known as long noncoding RNAs (lncRNAs) that are not translated into proteins. Historically, RNA has not been considered an active element in biological processes, and most disease research has not focused on them. Only in the past decade have scientists recognized that these RNA molecules can play critical roles in disease processes. Bhagat became interested in lncRNAs because they are involved in regulating diverse cellular processes and have been implicated in cancers.
To investigate the role of lncRNAs in tauopathies, the researchers started with skin cells from three people with a genetic tauopathy, each of whom carried a different mutation in the tau gene. Using molecular techniques, the researchers converted the skin cells into brain neurons that carry each of the three mutations. For comparison, they used a molecular technique known as CRISPR to correct the mutations in some of the skin cells before converting them into neurons. In this way, they were able to obtain human brain cells with and without tau mutations, which didn’t require using human brain tissue.